Vitaran Polynucleotides
by Vitaran
High-concentration (20mg/ml) PN bio-stimulator derived from purified Salmon Trout DNA. CE-marked for tissue stimulations and barrier repair.
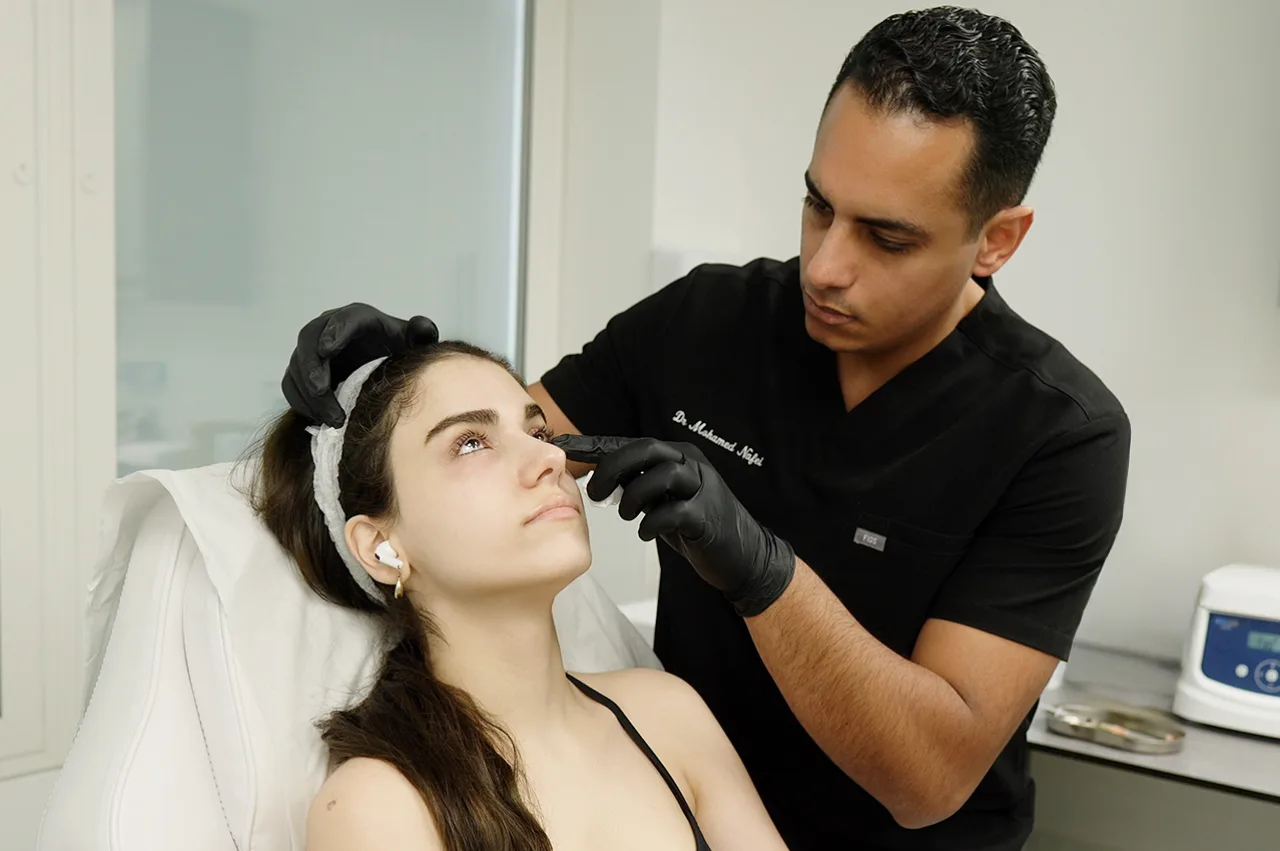
Treat Dark Circles, Fine Lines & Crepey Skin with CE-Marked Polynucleotide Therapy in London
Duration
30-45m30-45 Mins
Sessions
2-42-4 Recommended
Price From
£350£350
Downtime
MinimalMinimal (Bumps/Bruising)
The under-eye area is often one of the first places to show signs of ageing, including dark circles, fine lines, and thin, crepey skin. Polynucleotide (PN) injections offer a powerful regenerative solution specifically designed for this delicate periorbital zone. Unlike fillers that primarily add volume, PN treatments work biologically to improve skin health and quality from within.
At PRP London Clinic, our GMC-registered medical team uses CE-marked polynucleotide products including Vitaran Eyes, Nucleofill Soft Plus, and PolyPhil™ to target under-eye concerns effectively. Learn more about the benefits of Polynucleotide Injections for the full face and neck, or see our guide on Under-Eye Hollowing.
This procedure is part of our comprehensive Guide to Under-Eye Hollowing & Tear Trough Deformity.
Our polynucleotide under eye results demonstrate significant improvements in dark circles, skin texture, and fine lines. These before and after images show real patients treated at PRP London Clinic using CE-marked PN products.
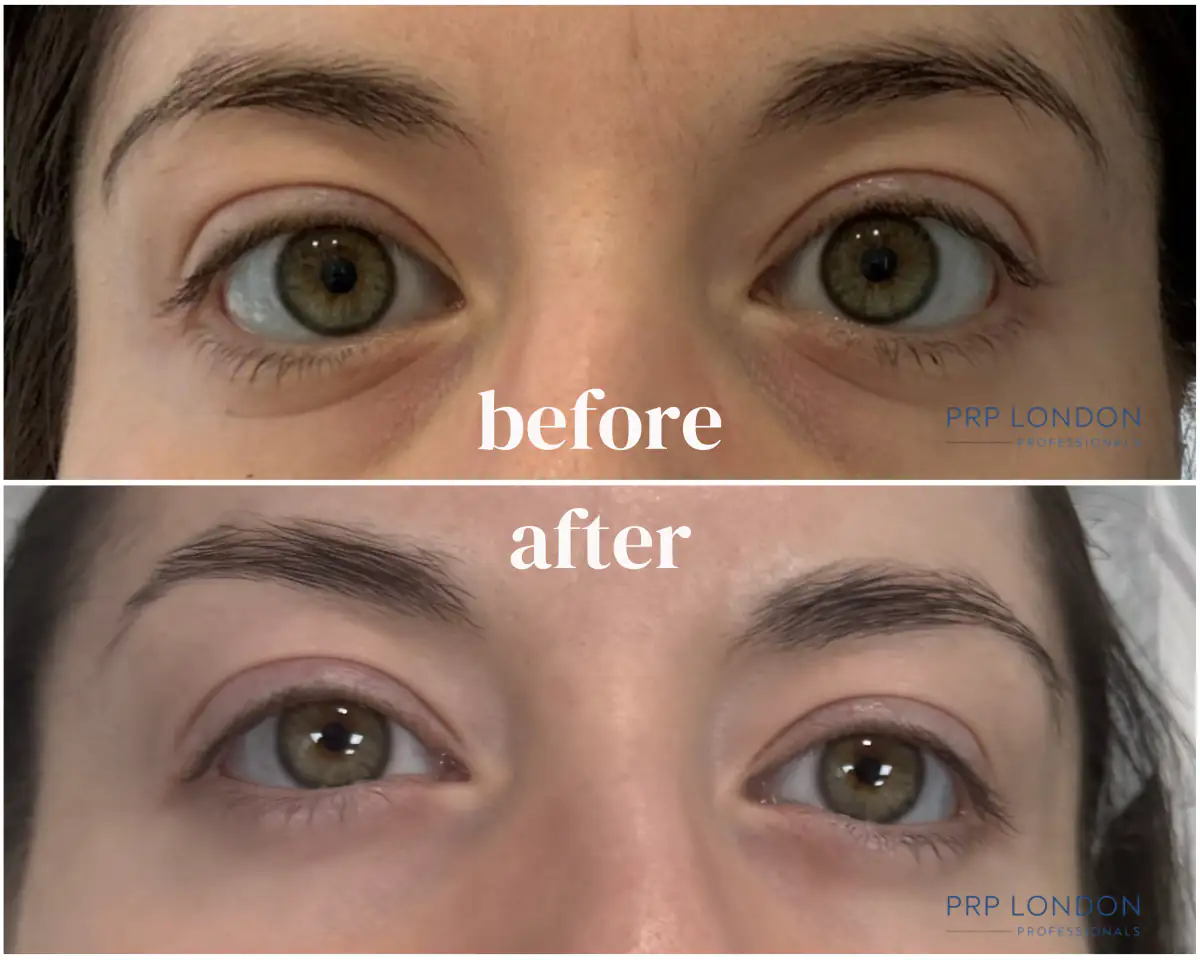
Macro-photography crop demonstrating observed improvement in under-eye hollowness, fine lines, and vascular shadows following a combined PolyPhil™ and PRF Bio-filler protocol. The periorbital area—containing the body's thinnest skin—serves as the most demanding test of any skin quality treatment. This combination approach addresses both skin quality (PN) and deeper structural concerns (PRF).
Treatment Course: 3 sessions of Polynucleotides (PolyPhil™) + 2 sessions of PRF Bio-filler. Results shown 10 weeks post-final session. Images taken under standard clinic photography conditions; minor variations in lighting/angle can occur. We use macro-photography to protect patient anonymity. Individual results vary.
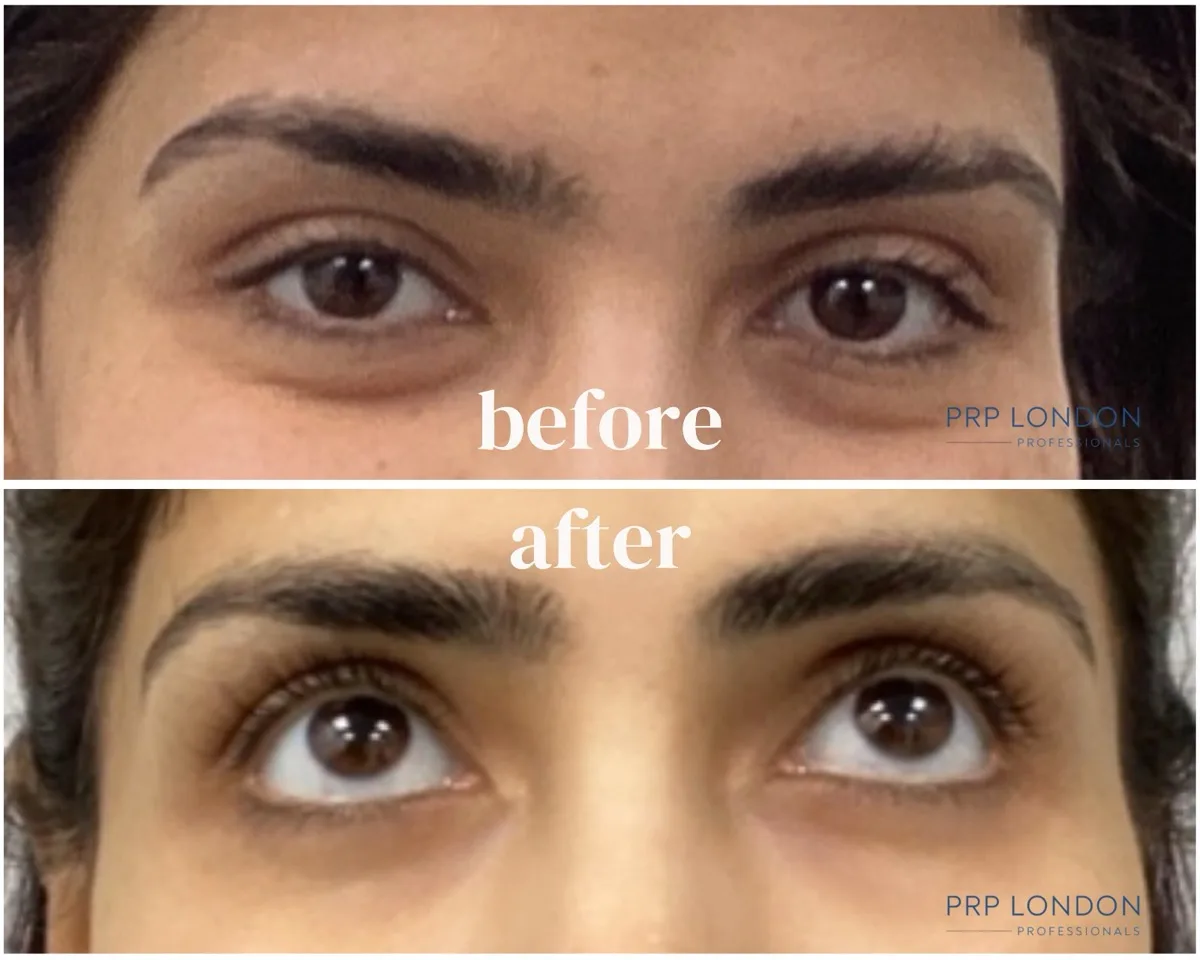
Periorbital rejuvenation demonstrating reduced pigmentation and improved tear trough contour after completing a PolyPhil™ and PRF Bio-filler combination protocol. The synergistic approach targets both the superficial skin changes responsible for dark circles and the deeper volume deficit contributing to shadowing. Results reflect ongoing tissue remodelling at the 4-week mark.
Treatment Course: 3 sessions of Polynucleotides (PolyPhil™) + 2 sessions of PRF Bio-filler. Results shown 4 weeks post-final session. Images taken under standard clinic photography conditions; minor variations in lighting/angle can occur. Individual results vary.
| Ideal Candidate | Not a Candidate |
|---|---|
| Dark circles caused by thin, translucent skin | Severe fat prolapse (true 'eye bags') requiring surgery |
| Fine lines and crepey skin texture | Malar edema or chronic puffiness (fluid retention) |
| Mild to moderate hollowing (Grade 1-2 tear trough) | Deep structural hollows requiring HA filler volume |
| Skin dehydration and dullness | Active skin infections or eczema around eyes |
| Those seeking gradual, natural improvement | Fish or seafood allergies (precautionary contraindication—although allergenic proteins are removed during purification) |
| Patients wanting to avoid synthetic fillers | Autoimmune diseases, pregnancy, or breastfeeding |
When injected into the tear trough and surrounding under-eye skin, polynucleotides are proposed to provide biological benefits. Suggested mechanisms include:
While these mechanisms are supported by preclinical and limited clinical data, the precise molecular pathways in human skin are not yet fully established.
Our Clinical Board selects from leading CE-marked products specifically formulated for the delicate periorbital area:
The choice depends on your specific concerns and our medical team's assessment during consultation.
Our medical team follows a precise clinical protocol to ensure optimal results and patient safety for polynucleotide under eye treatment.
Our GMC-registered doctor assesses your under-eye concerns, determines the grade of hollowing or skin thinning, and confirms your suitability. We follow a strict 'No Treatment if Not Suitable' policy—if your concerns are better addressed by surgery or HA fillers, we will advise accordingly.
The under-eye area is thoroughly cleansed and a medical-grade topical numbing cream is applied for 20-30 minutes to ensure complete comfort during the procedure.
Using either fine 30G needles or blunt-tipped micro-cannulas (to minimise bruising), our practitioner administers multiple micro-boluses of the polynucleotide product into the superficial dermal layers of the tear trough and periorbital skin.
Immediate review of injection sites. Small blebs (bumps) are expected and normal—these typically resolve within 24-48 hours as the product disperses into the tissue.
You'll receive comprehensive aftercare instructions including avoiding makeup for 12 hours, avoiding strenuous exercise for 24 hours, and sun protection. Most patients return to normal activities immediately.
Following consultation and cleansing, topical numbing cream is applied to the under-eye area for 20-30 minutes. Our practitioner then uses either a very fine needle or a cannula technique to administer multiple small injections of the chosen polynucleotide product into the superficial layers of the skin around and under the eye. The procedure typically takes 30-45 minutes. Small bumps under the skin immediately after injection are common and expected, usually resolving within 1-2 days.
Improvements are gradual as polynucleotides are proposed to support cellular activity:
Clinical outcomes vary based on age, skin condition, and individual regenerative response. Treatments are usually spaced 2-4 weeks apart. Maintenance sessions every 6-9 months may help preserve the results.
While both treat the under-eye area, they work differently and address different concerns:
| Feature | Polynucleotides | HA Tear Trough Fillers |
|---|---|---|
| Primary Action | Proposed biostimulation & skin support | Immediate volume replacement |
| Best For | Dark circles, fine lines, thin skin | Deep hollows, volume loss |
| Results Onset | Gradual over 4-12 weeks | Immediate |
| Duration | 6-12 months (reported) | 9-18 months |
| Risk of Lumps | Very low | Possible (Tyndall effect) |
They can sometimes be used complementarily—PN for skin support, HA for structural volume. Our medical team will advise which is more suitable for your specific concerns.
Polynucleotide under eye treatment is £350 per session. A course of 2-4 sessions is typically recommended for optimal results.
Considering treating face & neck too? View our full Polynucleotide Treatment page for complete pricing and combination packages.
Published clinical studies generally report good tolerability with predominantly mild, self-limiting side effects when performed by experienced practitioners; however, as with all injectable medical procedures, risks cannot be completely eliminated.
Common side effects (temporary):
Contraindications: Although allergenic proteins are removed during purification, patients with known fish or seafood allergies are treated as a precautionary contraindication. Other contraindications include autoimmune diseases, pregnancy or breastfeeding, and active skin infections.
Polynucleotide therapy is a medical procedure. Individual results vary based on age, skin condition, lifestyle factors, and individual biological response. CE-marked PN products meet regulatory compliance and safety standards, but this does not guarantee specific clinical outcomes. Results depend on individual regenerative capacity. A professional consultation with our GMC-registered medical team is required to determine suitability and set realistic expectations.
Evidence Note: Most published studies on polynucleotides are small or observational, with heterogeneity in products, protocols, and outcomes. More high-quality randomised controlled trials are required to definitively establish efficacy. These references are provided for educational purposes and do not replace individual clinical assessment.
Experience advanced polynucleotide under eye injections at PRP London Clinic in Harley Street and across our London locations. Our GMC-registered doctors specialise in periorbital rejuvenation using CE-marked Vitaran, Nucleofill, and PolyPhil products.
Address dark circles, fine lines, and tired-looking eyes with advanced Polynucleotide therapy. Book a consultation with our medical team at PRP London Clinic to see if polynucleotide under eye treatment is right for you.
False. PN is proposed to work biologically to support skin over weeks/months. Fillers provide immediate volume for hollows.
No, the small bumps (blebs) at injection sites are temporary and typically resolve within 24-48 hours as the product disperses.
Vitaran, Nucleofill, and PolyPhil are all high-quality CE-marked PN products. Our medical team selects the best option based on your specific needs.
For significant volume loss or deep hollows, HA Tear Trough Fillers are often more appropriate. PN is proposed to support skin quality in the area rather than add volume.
With topical anaesthetic applied beforehand, most patients report only mild discomfort. The needles used are very fine (30G).
| Treatment | Price |
|---|---|
| Polynucleotides Under Eyes Per session. Course of 2-4 sessions recommended for optimal results. | £350per treatment |
| Polynucleotides + Sunekos® Under Eyes Combination Combined protocol targeting under eye hollows, dark circles & skin quality. Polynucleotides stimulate deep tissue regeneration while Sunekos® rebuilds the extracellular matrix for comprehensive periorbital rejuvenation. | £550per treatment |
| Polynucleotides Under Eyes + Sunekos® Face Combination Dual-area protocol combining polynucleotide under eye rejuvenation with full-face Sunekos® ECM regeneration for a harmonious, refreshed appearance. | £650per treatment |
Prices are indicative. Please book a consultation for a personalised quote.
Comparison of Polynucleotide and Polydeoxyribonucleotide in Dermatology: Molecular Mechanisms and Clinical Perspectives
Kim ST · Pharmaceutics, 2025PMID: 40871045
Supports: A focused mechanistic comparison distinguishing PN (longer DNA fragments; ECM remodelling emphasis) from PDRN (shorter fragments; adenosine A2A receptor activation + salvage/de novo nucleotide pathways), with direct dermatology framing that can be cited for rationale statements in an under-eye PN page.
Limitations: Narrative review; clinical translation depends on heterogeneous products/protocols and the underlying evidence base rather than new primary trial data.
View on PubMed →Analysis of Skin Regeneration and Barrier-Improvement Efficacy of Polydeoxyribonucleotide Isolated from Panax ginseng (C.A. Mey.) Adventitious Root
Lee KS, Lee S, Wang H, et al. · Molecules, 2023PMID: 37959659
Supports: Demonstrates PDRN enhances keratinocyte/fibroblast proliferation and regenerative signalling, including adenosine A2A receptor agonism and downstream phosphorylation/signalling (FAK/AKT/MAPK), with effects also shown in a 3D skin model—useful to support biorevitalisation/skin quality claims (not volume replacement).
Limitations: Plant-derived PDRN and preclinical (cells/3D model) focus; not an under-eye clinical outcomes paper and may not map 1:1 to salmon-derived PN injectables used in aesthetic practice.
View on PubMed →Comparison of the effects of polynucleotide and hyaluronic acid fillers on periocular rejuvenation: a randomized, double-blind, split-face trial
Lee YJ, Kim HT, Lee YJ, et al. · Journal of Dermatological Treatment, 2022PMID: 32248707
Supports: Direct periocular (under-eye region) human RCT evidence: 27 subjects, split-face design, PN vs non-crosslinked HA, injected 3 sessions at 2-week intervals; reports safety with no serious adverse events and signals of improvement (including higher improvement rates in roughness and pore volume for PN).
Limitations: Small sample; outcomes are periocular rejuvenation/skin quality rather than true tear-trough volumisation; follow-up constraints limit certainty on durability.
View on PubMed →*PRP London Clinic provides these references for educational purposes. Our Clinical Board regularly reviews emerging peer-reviewed literature to ensure our protocols align with the latest advancements in regenerative medicine.
Your treatment will be performed by one of our qualified medical professionals
Scroll to see all
Book your consultation today and take the first step towards your transformation.
Ruby K
19 Jan 2026
Norin Hanifi
19 Jan 2026
Dominique Heslop
13 Jan 2026
lloyda221
06 Jan 2026
Mane Authority
06 Jan 2026
Victor Contreras Guerra
4 months ago
A A
5 months ago
harris jan
3 months ago
Independent Patient Feedback
Read all verified stories →As a GMC-regulated practice, a mandatory consultation is required for all new patients to assess suitability and clinical need. This treatment may not be appropriate for everyone. All procedures are performed by GMC-registered doctors whose credentials can be verified on the official GMC register.
by Vitaran
High-concentration (20mg/ml) PN bio-stimulator derived from purified Salmon Trout DNA. CE-marked for tissue stimulations and barrier repair.
by PolyPhil
High Purification Polynucleotides (PN-HPT®) derived from Trout DNA for deep skin biorevitalisation.
by Nucleofill
High-viscosity bio-restructuring gel composed of long-chain salmon-derived polynucleotides.
This technical specification is provided for informational purposes only. All medical devices and substances are used exclusively by GMC-registered practitioners at PRP London Clinic. Individual results may vary.